Analysis of quality changes and specific spoilage organisms in channel catfish during cold storage
-
摘要:
为分析鮰鱼在低温贮运期间品质变化的规律并确定其优势腐败菌,该研究将鮰鱼肉块分别置于4和10 ℃条件下冷藏,通过测定感官指标、质构特性、色度、pH值、菌落总数(total viable count, TVC)及挥发性盐基氮(total volatile basic nitrogen, TVB-N)含量等指标,系统分析其品质变化规律。同时,采用高通量测序和16S rDNA技术鉴定分析鮰鱼中的微生物组成,并对优势腐败菌的致腐能力进行评估。结果表明,冷藏期间鮰鱼的pH值呈现先下降后上升的趋势,硬度、咀嚼度及红绿值a*均呈下降趋势,且TVB-N含量与TVC呈正相关。其中4 ℃条件下鮰鱼的腐败速率较慢,感官品质保持较好,微生物分析显示,相对丰度较高的气单胞菌属(Aeromonas)(28.74%)和希瓦氏菌属(Shewanella)(6.34%)致腐能力也最强,是鮰鱼冷藏期间的优势腐败菌。综上所述,鮰鱼4 ℃条件下冷藏时的新鲜度和品质保持较好,气单胞菌属和希瓦氏菌属是导致鮰鱼腐败的主要菌属。该研究为基于菌群结构演替规律的淡水鱼品质控制技术开发提供了一定的理论依据和数据支持。
Abstract:Channel catfish is the most numerous catfish species in the world. This study aims to analyze the dynamics of quality and bacterial composition in channel catfish subjected to various low-temperature storage. The research also revealed the correlation between the evaluation metrics and the predominant spoilage bacteria. Specifically, the catfish was selected and then stored under refrigeration at 4 and 10 °C. Various parameters included sensory metrics, texture, color, pH value, total viable count (TVC), and the total volatile base nitrogen (TVB-N). High-throughput sequencing was employed to determine the microbial community composition of catfish. Traditional sieving was utilized to isolate and identify the microorganisms. While the spoilage capacity and growth kinetics were utilized to characterize the dominant spoilage species. The results indicated that the pH changes of catfish exhibited the initial decline and then increased, with the prolongation of low-temperature storage time. Additionally, the TVB-N, TVC, lightness (L*), yellow-blue value (b*), and Whiteness (W) demonstrated an increasing trend, whereas the hardness, chewiness, and red-green value (a*) displayed a decreasing trend. Furthermore, The TVB-N content exhibited a significant positive correlation with the TVC. There was a correlation coefficient of 0.95 (P<0.05). The decay rate of catfish was slower under the condition of 4 °C. The sensory quality was maintained, in terms of the various indicators. Microbiological analyses found that Firmicutes, Bacteroidota, and Proteobacteria were the dominant phylum associated with catfish spoilage. The corruption of catfish meat involved a total of 19 families, more than 20 genera, and more than 30 bacterial species. According to the genus level, the predominant genera with the high relative abundance of the catfish were identified as Myroides sp. (39.68%), Aeromonas sp. (28.74%), Acinetobacter sp. (16.33%) and Shewanella sp. (6.34%). A total of six bacterial strains were screened, including two strains of Pseudomonas, one strain of Shewanella, two strains of Aeromonas veronii, and one strain of Acinetobacter johnsonii. Among them, the spoiled catfish muscle was taken as the raw material. The screening data was consistent with the microbial diversity on the channel catfish. The growth kinetics was used to determine the two strains with the strongest decay at different temperatures. Furthermore, the lag phase (λ) was extended significantly, as the incubation temperature decreased. While the maximum specific growth rate (μmax) was reduced significantly. Notably, the μmax of Shewanella baltica consistently exceeded that of Aeromonas veronii. Both strains shared excellent growth at low temperatures, where Shewanella veronii was more suitable for the low-temperature adaptation. The yield factor (YTVB-N/CFU) revealed that the spoilage potential greatly varied among the six identified spoilage bacteria strains. Aeromonas veronii and Shewanella baltica exhibited the most pronounced spoilage potential, whereas Acinetobacter johnsonii had relatively limited spoilage activity. The findings can offer valuable insights for future investigations into the spoilage mechanisms of channel catfish. A theoretical foundation and data support were also provided for the quality control technologies for the freshwater fish, according to the succession patterns of microbial communities. A great contribution was also made to the low-temperature sustainable storage and transportation of freshwater fish.
-
Keywords:
- channel catfish /
- cold storage /
- quality /
- specific spoilage organisms
-
0. 引 言
鮰鱼,亦称沟鲇,属于鮎形目、鮰科鱼类,是中国具有较高经济价值的水产品之一。1984年由湖北省水产科学研究所引进后[1],其因适应性强、生长快、产量高、肉质鲜嫩且价格低廉等诸多优势[2],迅速成为淡水食用鱼中的上品。鮰鱼富含脂肪、蛋白质和水分[3],在冷藏过程中极易受到微生物污染而发生腐败,影响其感官品质和经济价值。在贮藏过程中,微生物和内源酶的共同作用导致蛋白质降解为多肽,并进一步降解为氨基酸、氨和部分挥发性有机物[4],这些含氮代谢产物导致水产品的感官特性(硬度、咀嚼度、异味和颜色)的下降以及有害物质的积累[5],严重降低了其商品价值和食用安全性。此外,市售新鲜鮰鱼或鮰鱼片通常采用低温冷藏(4 ℃)的方法延长货架期,而在实际冷链贮运过程中,温度可能会在4~10 ℃之间波动。这种温度波动可能会影响鮰鱼的品质变化,加速微生物的生长和代谢活动,从而缩短其货架期。
微生物的生长及代谢活动是水产品腐败变质的主要原因。研究表明,水产品腐败的复杂过程中占据主要地位的只有少数特定的微生物群体,即优势腐败菌(specific spoilage organisms, SSOs)。革兰氏阴性嗜冷微生物包括希瓦氏菌、气单胞菌、假单胞菌、肠杆菌和弧菌等是水产品中常见的SSOs。LI等[6]发现冷藏鲷鱼的优势腐败菌为假单胞菌、希瓦氏菌和气单胞菌;周慧等[7]发现虹鳟鱼贮藏过程的特定腐败菌为希瓦氏菌属;柴庭庭等[8]发现不同腐败菌接种于大黄鱼肉会产生不同的不良风味物质,且希瓦氏菌属是真空包装大黄鱼贮藏期间的优势腐败菌属;刘洋帆等[9]发现嗜水气单胞菌对4 ℃冷藏鲣鱼的致腐产胺能力最强。这些研究表明,SSOs因水产种类、捕获季节和贮藏环境的不同而有显著差异。而且SSOs会产生异味,同时加速水产品腐败变质,不仅降低了鱼肉的营养价值,还可能对消费者健康构成潜在威胁。鮰鱼作为市场消费和冷链运输中的主要淡水鱼品种,但目前关于鮰鱼在冷藏过程中微生物群落结构及其优势腐败菌的研究报道较少。因此,研究鮰鱼在冷藏过程中品质变化规律及优势腐败菌,对于改进鮰鱼低温贮运品质具有重要意义。
本研究以鮰鱼为试验材料,探究其在4和10 ℃(冷链运输中最常用的温度)冷藏过程中微生物群落、理化性质和感官品质的规律及相关性,并分离鉴定出低温腐败菌,利用挥发性盐基氮产量因子(YTVB-N/CFU)分析鮰鱼中腐败菌的致腐能力,并探究其在低温条件下的生长情况,以期为鮰鱼贮运保鲜关键技术提供理论基础。
1. 材料与方法
1.1 材料与试剂
鮰鱼:每条平均质量1.60 kg,购自于当地水产市场(中国大连);氯化钠、硼酸、盐酸(均为分析纯),天津市大茂化学试剂厂;甲基红、溴甲酚氯(均为分析纯),大连博诺有限公司;氧化镁(分析纯),北京宝希迪有限公司;琼脂、LB(Luria-Bertani)培养基、PCA(plate count agar)培养基(生物试剂),青岛高科技工业园海博生物技术有限公司;基因组DNA提取试剂盒,天根生化科技(北京)有限公司。
1.2 仪器设备
Jipad-20 拍打式无菌均质器,上海旌派仪器有限公司;Scan4000 全自动超高清菌落计数器,法国Interscience公司;Micro 17R 冷冻高速离心机,美国Thermo Scientific公司;K9840 自动凯氏定氮仪,海能仪器股份有限公司;YZB/GER
1700 -2013 冷冻高速离心机,赛默飞世尔科技(中国)有限公司;pH计,梅特勤-托利多仪器(上海)有限公司;Infinite M200多功能酶标仪,瑞士TECAN公司;Stable Micro Systems TA.XT.plus 质构仪,超技仪器有限公司;HunterLab UltraScan PRO 测色仪,上海信联创作电子有限公司;synergy HTX酶标仪,GeneCompang基因有限公司。1.3 试验方法
1.3.1 原料与处理
将鲜活鮰鱼宰杀后,沿脊柱切开去除内脏,用清水将其冲洗干净,装于自封袋中,置于4和10 ℃下恒温贮藏。
1.3.2 感官评价
参照杨永安等[10]的方法,从鮰鱼的外观、气味、质地和黏液4个方面进行评价,各项指标满分为15分。感官评分标准如表1。
表 1 感官评定指标Table 1. Sensory evaluation index评分项目
Scoring item感官指标
Sensory index分数
Score外观
Appearance色泽均匀,表面富有光泽 11~15 色泽较暗淡,基本无光泽 6~10 暗淡无光泽 1~5 气味
Smell有较淡的鲜美鱼腥味,无不良气味 11~15 腥味明显,略有臭异味 6~10 有强烈的腥臭味 1~5 质地
Texture肌肉坚实,有弹性,外形保持完好 11~15 肌肉松软,弹性较差,外形保持较完好 6~10 肌肉软烂,无弹性,外形保持较差 1~5 黏液
Mucus透明,无黏液 11~15 较少黏液,乳白色 6~10 较多黏液,黄色 1~5 1.3.3 质 构
参考卜俊芝等[11]的方法稍作调整。将鮰鱼样品切成20 mm×20 mm×10 mm大小后,用质构仪测定鱼肉的质构特性。探头型号:P50;模式:全质构模式(texture profile analysis, TPA)。测定参数:测前速度1 mm/s、测试速度2 mm/s、测后速度5 mm/s。
1.3.4 色 度
采用测色仪对鱼肉组织样品进行测定,记录亮度L*、红绿值a*、黄蓝值b*值,白度W的计算式为[12]
$$ {}{W}{=100-}\sqrt{{{(100-}{{L}}^{{*}}{)}}^{{2}}+{{{a}}^{{*}}}^{{2}}+{{{b}}^{{*}}}^{{2}}} $$ (1) 1.3.5 pH值
参照GB 5009.237-2016《食品安全国家标准 食品pH值的测定》中的方法测定。
1.3.6 菌落总数
参照GB 4789.2-2022《食品安全国家标准 食品微生物学检验 菌落总数测定》中的方法测定。
1.3.7 挥发性盐基氮含量
参照GB 5009.228-2016《食品安全国家标准 食品中挥发性盐基氮的测定》中的方法测定。取10.00 g鱼肉匀浆后,采用半自动凯氏定氮仪测定挥发性盐基氮(total volatile basic nitrogen, TVB-N)含量。
1.3.8 微生物多样性
取0.50 g鮰鱼肌肉组织样本,采用基因组DNA试剂盒,按照说明书完成核酸的提取。使用酶标仪对核酸进行浓度检测,利用通用引物27F(5'- AGRGTTTGATYNTGGCTCAG-3')和1492R(5'-TASGGHTACCTTGTTASGACTT-3')扩增全长16S rDNA基因[13]。聚合酶链式反应(polymerase chain reaction,PCR)条件:95 ℃预变性2 min;98 ℃变性10 s,55 ℃退火30 s,72 ℃延伸1 min 30 s,扩增25个循环;72 ℃稳定延伸2 min;在4 ℃下保存。扩增后PCR产物使用浓度为1.8%的琼脂糖凝胶进行电泳检测,符合条件的样品进行混样[14]。
使用建库试剂盒(SMRTbell Template Prep Kit)对混合产物进行修复及连接,最终使用磁珠(AMpure PB)纯化回收得到上机文库;使用结合试剂盒(PacBio Binding kit)对上机文库进行上机前的结合,使文库结合引物(primer)及DNA聚合酶(DNA polymerase);将最终的反应产物进行磁珠(AMpure PB)纯化后置于测序仪(SequelII)上进行测序[15-16]。
1.3.9 腐败菌的分离鉴定
取5.00 g冷藏(4 ℃,7 d)的鮰鱼样品,加入45 mL无菌生理盐水拍打均质,制成菌悬液。将菌悬液梯度稀释,取100 μL适当浓度梯度的菌悬液涂布于LB固体培养基中,在30 ℃恒温箱中培养24 h。挑选不同形态的单菌落,用平板划线法分离纯化菌株[7]。同时,将所筛选的菌株接种于新的LB固体培养基上,置于4 ℃培养,以筛选低温腐败菌。将4 ℃培养生长的单菌落接种到LB液体培养基中,摇床(30 ℃,150 r/min)培养后,采用甘油管藏法保存于−80 ℃冰箱备用。利用普通光学显微镜观察菌体形态。菌株基因组DNA的提取采用玻璃珠破碎法[17]。利用16S rDNA通用引物进行PCR扩增,产物经琼脂糖凝胶电泳检测后送至生工生物工程(上海)股份有限公司进行测序,测序结果通过NCBI网站(https://www.ncbi.nlm.nih.gov/)上的BLAST程序进行对比分析。
1.3.10 菌悬液的制备
参考柴庭庭等[8]的方法稍作修改。将分离得到的腐败菌在LB固体培养基活化两次后,接种到LB液体培养基,摇床振荡培养(30 ℃,150 r/min)至对数期。将菌液离心(8 000 r/min,10 min)收集菌体,用无菌生理盐水将收集的菌体进行重悬,并将菌液浓度调整为106 CFU/mL,以便于后续试验。
1.3.11 无菌鱼块的制备与接种
参照高天麒等[18]的方法制备无菌鱼块。将鲜活鮰鱼宰杀,沿脊柱切开去除内脏后,用无菌水将其冲洗干净。使用无菌手术刀将鮰鱼的背部肉切割成50.00 g的肉块。首先将鱼块在75%的酒精中浸泡1~2 min,以确保酒精充分发挥杀菌作用,随后用无菌水清洗,以去除残留的酒精和可能存在的微生物污染,最后将鮰鱼样品置于超净台中紫外照射15~20 min。测定鱼块的菌落总数小于102 CFU/g,可视为无菌。将处理好的无菌鱼块放入调好浓度的菌悬液中浸泡5 min后,取出置于无菌滤纸上晾干,以便后续致腐能力试验。
1.3.12 致腐能力
参考郑瑞生等[19]的方法测定不同微生物的致腐能力,将1.3.11中接种腐败菌的鱼块密封于无菌袋中,4 ℃贮藏10 d后取出,测定样品菌落总数(total viable count, TVC)和TVB-N含量,按式(2)计算。
$$ {{Y}}_{\rm{TVB-N/CFU}}=\frac{{{C}}_{{S}}-{{C}}_{{0}}}{{{N}}_{{S}}-{{N}}_{{0}}} $$ (2) 式中C0为初始的TVB-N含量,mg/100 g;CS为结束时的TVB-N含量,mg/100 g;N0为初始的菌落总数,CFU/g;NS为结束时的菌落总数,CFU/g。
1.3.13 SSOs的生长动力学
将SSOs活化接种于10 mL的LB液体培养基中,纯化传代2次,取1.5 mL菌液调节浓度使得OD600值(菌液在600 nm波长处的吸光值)在0.6~0.8之间,将调节后的菌液按2%的接种量接种于50 mL的LB液体培养基中,分别置于4、10、15和30 ℃的培养箱中培养,每隔一定时间取样测定其OD600值,记录数据,绘制不同温度条件下的生长曲线。
腐败菌生长动力学模型采用修正的Gompertz方程如式(3)所示[20]。
$$ N_t=N_0+(N_{\mathit{\mathrm{\mathit{\max}}}}-N_0)\times\exp\left\{-\exp\left[\frac{\mu_{max}\times2.718}{N_{max}-N_0}\times(\lambda-t)+1\right] \right\}$$ (3) 式中t为培养时间,h;Nt为t时刻的OD600值;N0为初始OD600值;Nmax为最大OD600值;μmax为最大比生长速率,h–1;λ为延滞期,h。
1.3.14 数据统计与分析
SPSS Statistics 26的统计软件对试验结果进行显著性分析。Origin 2021绘制折线图、柱形图、雷达图和Pearson相关性分析。本研究中每组试验包含3组平行,试验数据均以平均值±标准差的形式呈现。
2. 结果与分析
2.1 感官评价
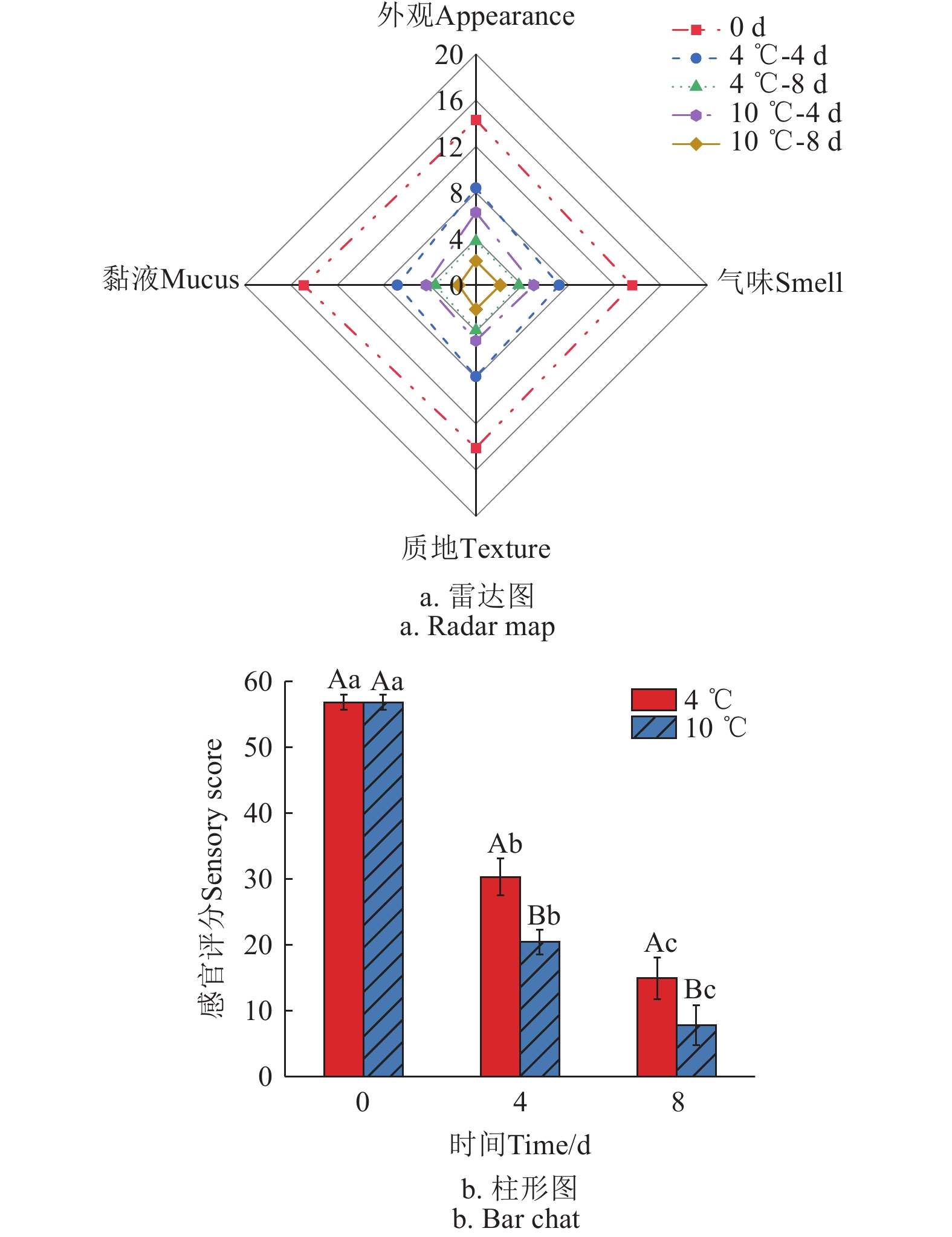
消费者可以通过色泽、气味、质地等感官特征来评估水产品的新鲜度,这些感官指标是判断鮰鱼新鲜程度最直接且有效的方法。感官评分低于30分表明鮰鱼在感官特性方面已经不能满足消费者的期望和购买标准[21]。如图1所示,新鲜鮰鱼的初始感官评分高达56.8分(满分60分),肌肉色泽均匀,明亮自然,有较轻的腥味,无其他不良气味,组织坚实有弹性,肉质良好。随着贮藏时间的增加,鮰鱼的感官评分分值明显下降,色泽暗淡,无光泽,出现腥臭味,组织松软,黏液发黄,其中4 ℃下的感官评分显著高于10 ℃下的感官评分,第4天时,10 ℃下的鮰鱼肉感官评分已经降至20.4分,不可食用,而4 ℃条件下的感官评分为30.3分,尚接近正常水平。这种现象主要是酶促反应、微生物活动、蛋白质降解和脂肪氧化共同作用的结果[22]。
![]() 图 1 鮰鱼在不同温度贮藏期间的感官评分注:大写字母不同表示相同贮藏时间内,不同贮藏温度差异显著;小写字母不同表示相同贮藏温度,不同贮藏时间差异显著(P<0.05),下同。Figure 1. Sensory scores of channel catfish during storage at different temperaturesNote: The difference of capital letters means that the difference of storage temperature is significant in the same storage time. Different lowercase letters indicate the same storage temperature, and the difference is significant for different storage times (P < 0.05), the same below.
图 1 鮰鱼在不同温度贮藏期间的感官评分注:大写字母不同表示相同贮藏时间内,不同贮藏温度差异显著;小写字母不同表示相同贮藏温度,不同贮藏时间差异显著(P<0.05),下同。Figure 1. Sensory scores of channel catfish during storage at different temperaturesNote: The difference of capital letters means that the difference of storage temperature is significant in the same storage time. Different lowercase letters indicate the same storage temperature, and the difference is significant for different storage times (P < 0.05), the same below.2.2 质构特性
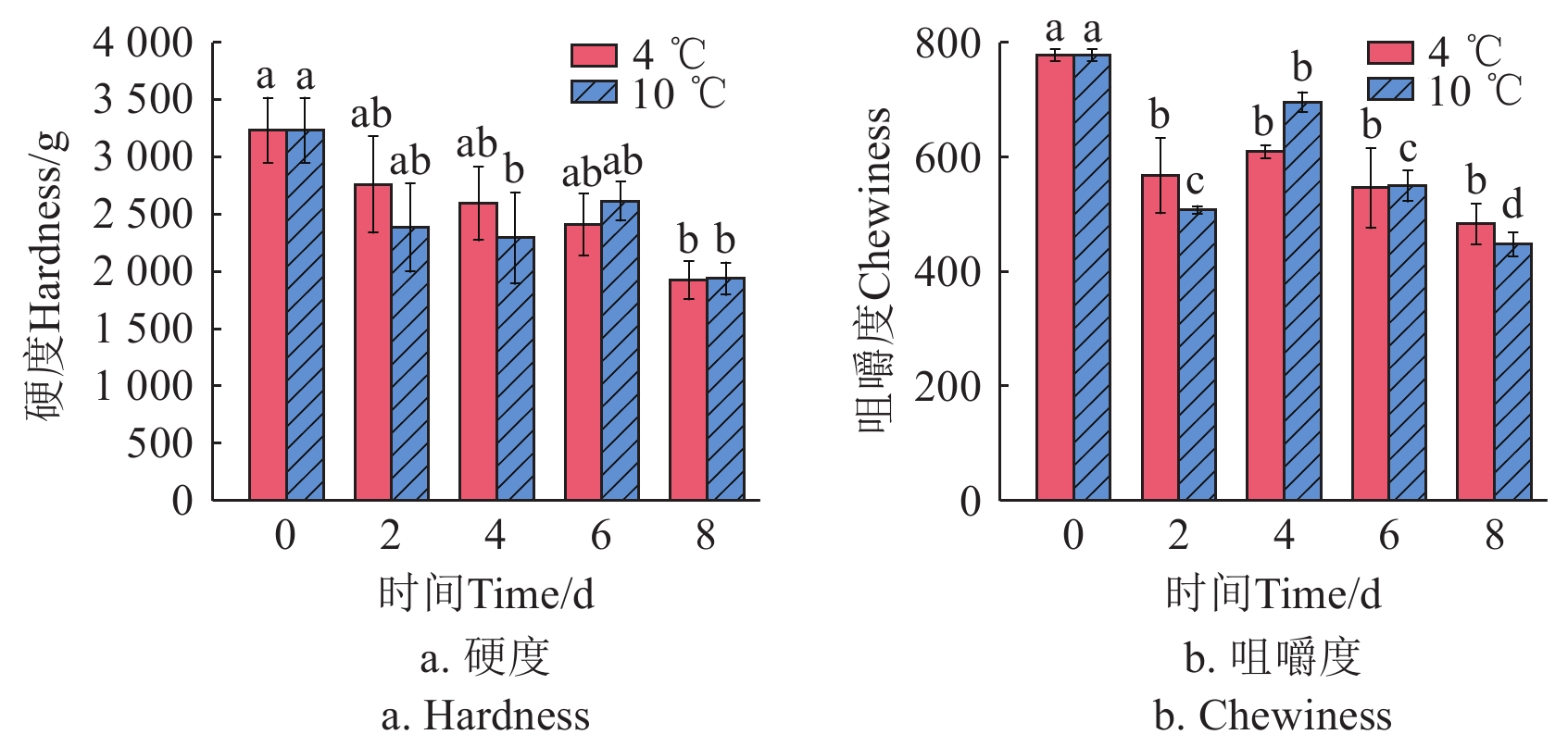
质构特性能够在一定程度上反映鱼肉的感官品质[23]。在4和10 ℃贮藏条件下,鮰鱼肌肉的硬度(图2a)和咀嚼度(图2b)呈波动下降的趋势,可能是由于在冷藏期间蛋白质变性,肌原纤维结构被破坏,肌肉细胞间结合力下降。LAN等[24]发现太平洋白对虾在贮藏过程中硬度、弹性和咀嚼度明显降低,这与本研究结果一致。其中,4和10 ℃条件下贮藏8 d后的肌肉咀嚼度相比于新鲜鮰鱼分别下降了37.88%和42.50%,在4 ℃贮藏条件下,鮰鱼肌肉的咀嚼度更佳,这可能是由于该温度减缓了鱼肉中肌动球蛋白的氧化变性和降解速率[22]。
2.3 色 泽
颜色是决定鮰鱼在市场上可接受度的重要品质特征。如表2~3所示,4和10 ℃贮藏条件下,鮰鱼肌肉的a*、b*、L*和W的变化趋势相近。随着贮藏时间的延长,a*值呈下降趋势,且始终为负值,表明鮰鱼肉红度下降,这可能是由于肌肉组织被氧化以及大量微生物的繁殖促进了高铁肌红蛋白的形成,高铁肌红蛋白呈褐色,使得a*值下降[25]。
表 2 鮰鱼在4 ℃下贮藏期间色泽的变化Table 2. Changes of color of channel catfish during storage at 4 ℃时间
Time/dL* a* b* W 0 49.56±2.90b −2.37±0.20a 0.46±0.33b 49.50±2.89b 2 49.81±1.48b −3.16±0.24b 0.74±0.60b 49.70±1.47b 4 50.37±1.00b −3.49±0.25bc 2.34±0.50a 50.19±0.98b 6 51.70±3.17ab −3.68±0.34c 2.38±0.63a 51.49±3.16ab 8 53.46±1.33a −4.25±0.44d 2.63±0.76a 53.19±1.37a 注:L*表示亮度,a*表示红绿值、b*表示黄蓝值,W表示白度,下同。 Note: L* represents lightness, a* represents the red-green value, b* represents the yellow-blue value, and W represents the whiteness, the same below. 表 3 鮰鱼在10 ℃下贮藏期间色泽的变化Table 3. Changes of color of channel catfish during storage at 10 ℃时间
Time/dL* a* b* W 0 49.56±2.90abc −2.37±0.20a 0.46±0.33d 49.50±2.89b 2 48.05±3.54bc −2.53±0.75ab 2.14±1.05c 47.93±3.50ab 4 46.97±0.84c −3.40±0.37bc 2.81±1.12bc 46.78±0.84ab 6 50.57±2.08ab −3.77±0.75c 3.72±1.01ab 50.27±2.07a 8 51.44±1.43a −3.85±1.10c 5.01±1.28a 51.00±1.42a 2种温度条件下的a*值相差不大,表明温度对红绿值的影响较小。在整个贮藏过程中,b*值呈上升趋势,表明鮰鱼肉黄度上升,4 ℃贮藏下的鮰鱼肌肉b*值较低,这可能是由于鮰鱼肉中的脂肪随贮藏时间发生了氧化,且4 ℃下脂质氧化程度更低。随着时间的推移,L*值和W整体呈上升趋势,表明鱼肉亮度和白度逐渐增大。贮藏期间颜色的变化可能是由于蛋白质和脂质的氧化和降解,使光的吸收和散射发生了改变,同时产生了一些影响颜色的氧化产物,如氢过氧化物、酮、酸和醛[26]。
2.4 pH值
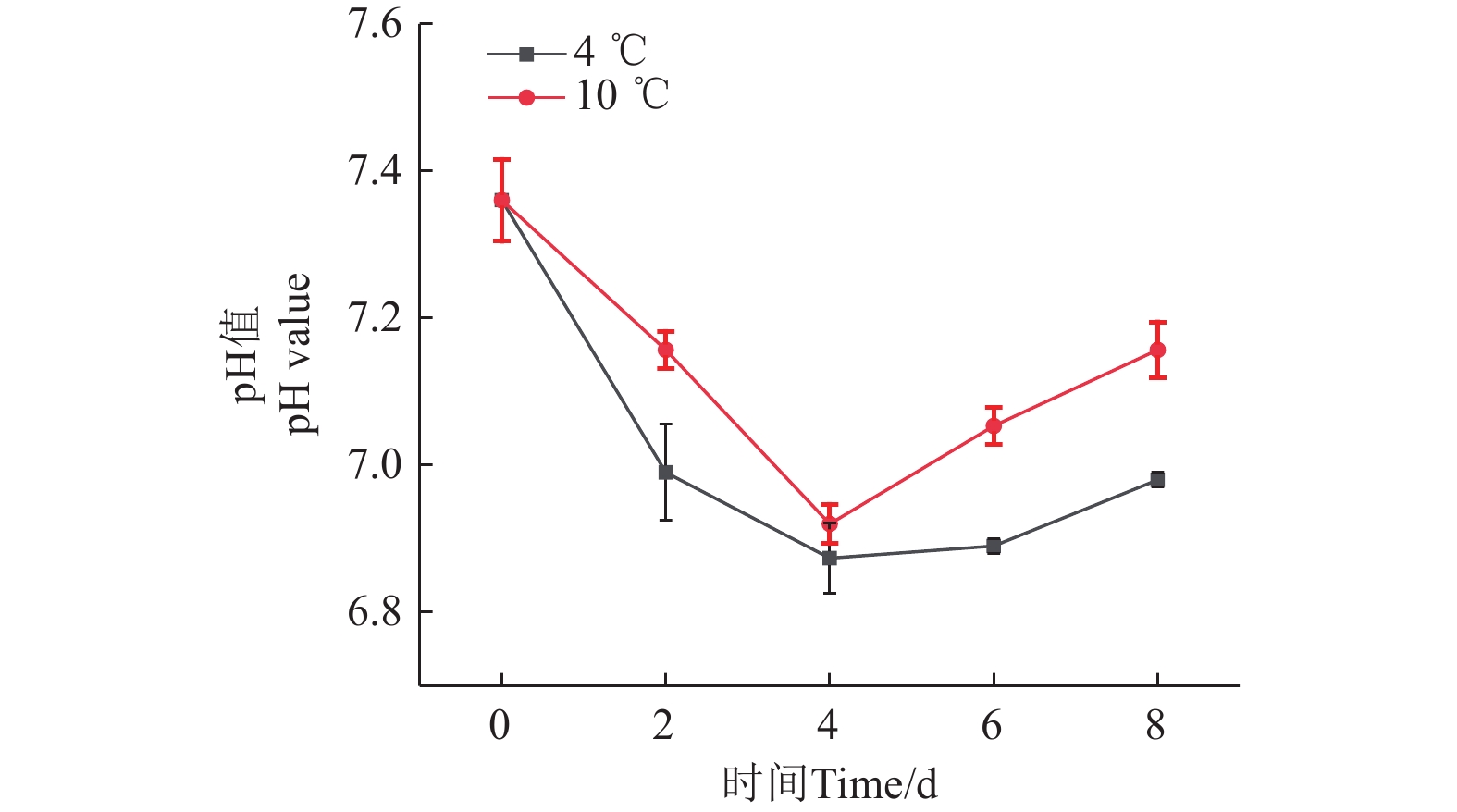
pH值是衡量鮰鱼新鲜度的一个重要指标[27]。如图3所示,4和10 ℃贮藏条件下,鮰鱼肌肉的pH值均呈先下降后上升的变化趋势。第0天时,pH值为7.36,在4和10 ℃条件下贮藏4 d后,pH值下降至最低点,分别为6.87和6.92,这是由于糖原在酵解过程中产生了乳酸、磷酸等酸性代谢产物[28];pH值达到最低值后,由于蛋白质在内源酶的作用下发生分解,产生挥发性氨等碱性物质[29],使得pH值上升。4 ℃条件下,鮰鱼pH值上升速度相对较慢,这可能是由于低温抑制了微生物的生命活动,内源酶的活性变低,蛋白质分解速度更慢,碱性物质生成更少。
2.5 菌落总数
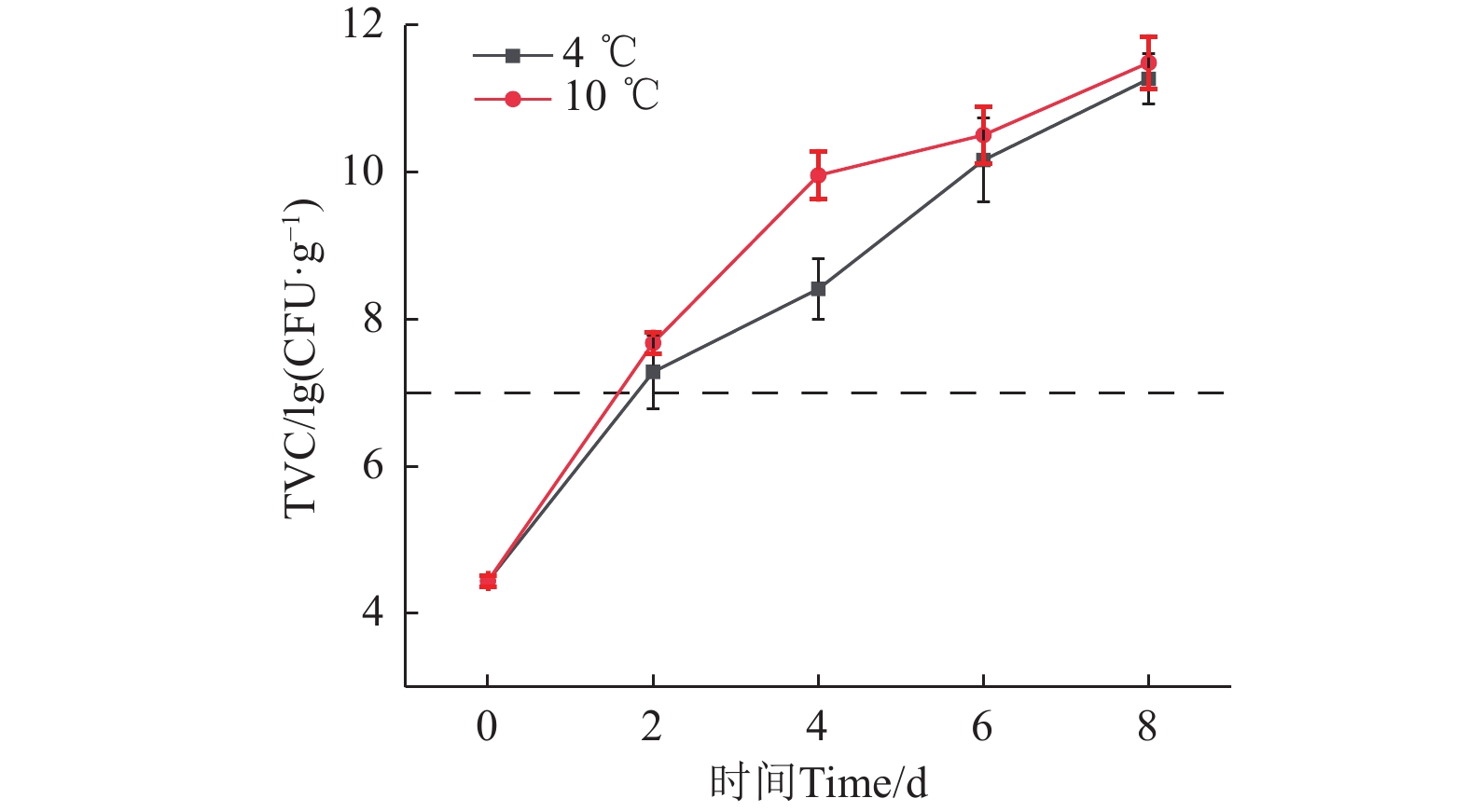
微生物的代谢活动是导致水产品变质的主要原因之一。如图4所示,即宰杀后,新鲜鮰鱼的菌落总数( TVC)为4.44 lg(CFU/g),2种温度下贮藏2 d后均超过国际微生物标准委员会规定的水产品微生物限量标准(≤7 lg(CFU/g)),这是因为鮰鱼肉中含有丰富的营养物质,为微生物的生长和繁殖提供了条件,导致鱼肉腐败变质[30]。
不同低温贮藏条件下,鮰鱼肉的TVC均呈上升趋势,2种温度贮藏8 d时的TVC接近,但4 ℃条件下的TVC增长相对较慢,且在贮藏过程中TVC低于10 ℃,表明低温可以有效抑制微生物的活性,从而降低微生物增长和繁殖的速率。
2.6 TVB-N含量
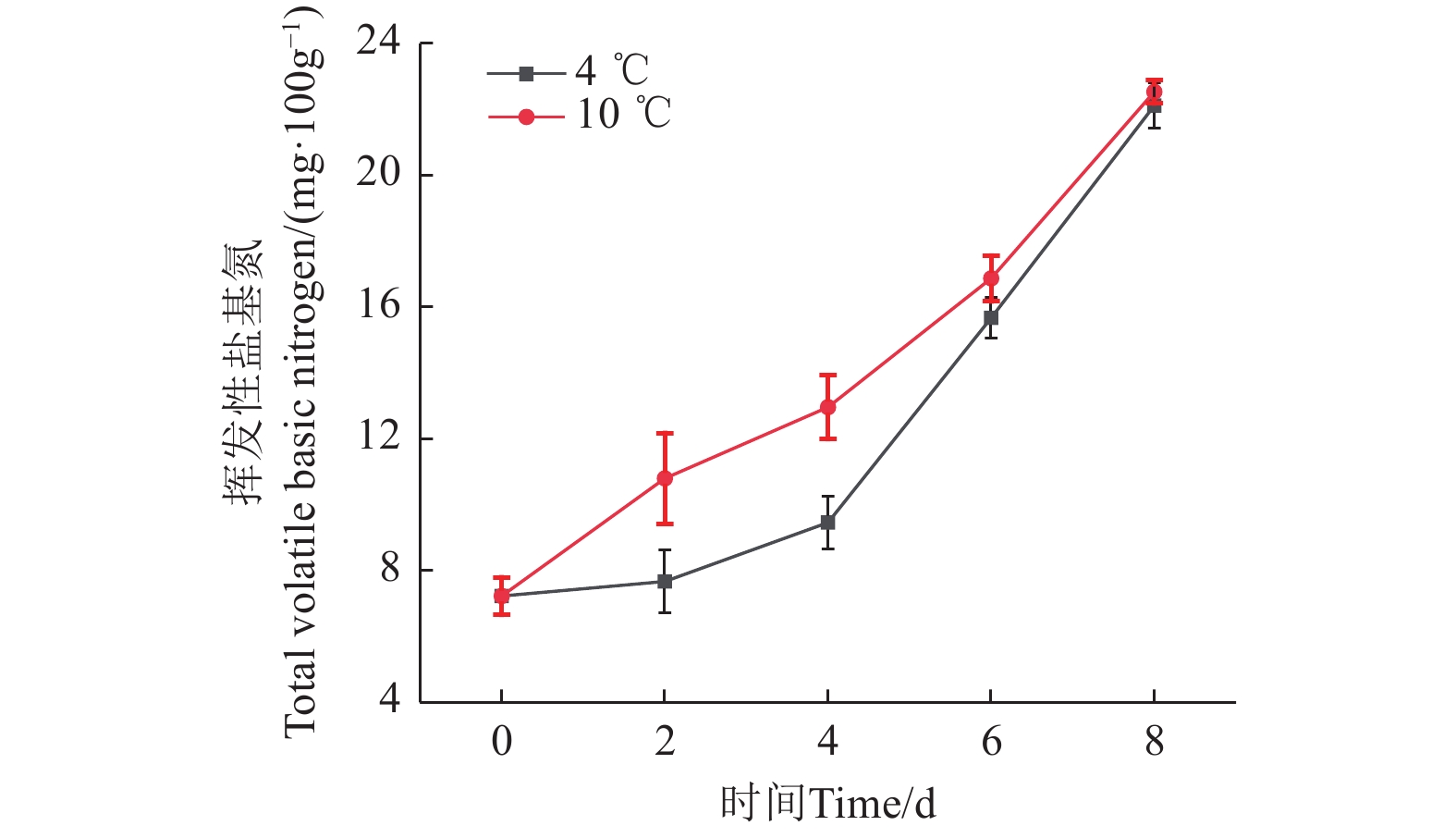
挥发性盐基氮(TVB-N)含量是衡量水产品新鲜度的一项关键指标,它反映了在微生物和酶的共同作用下,蛋白质分解生成挥发性含氮化合物的总量。如图5所示,新鲜鮰鱼肌肉中的TVB-N含量为7.23 mg/100 g,在冷藏前期,TVB-N含量上升较为缓慢。然而,在10 ℃贮藏条件下,其含量上升速率加快,贮藏4 d时已达到12.96 mg/100 g,而在4 ℃贮藏条件下的TVB-N含量为9.45 mg/100 g,增长较为缓慢,这表明低温能有效抑制微生物活性,延缓腐败进程[31]。
2.7 Pearson相关系数
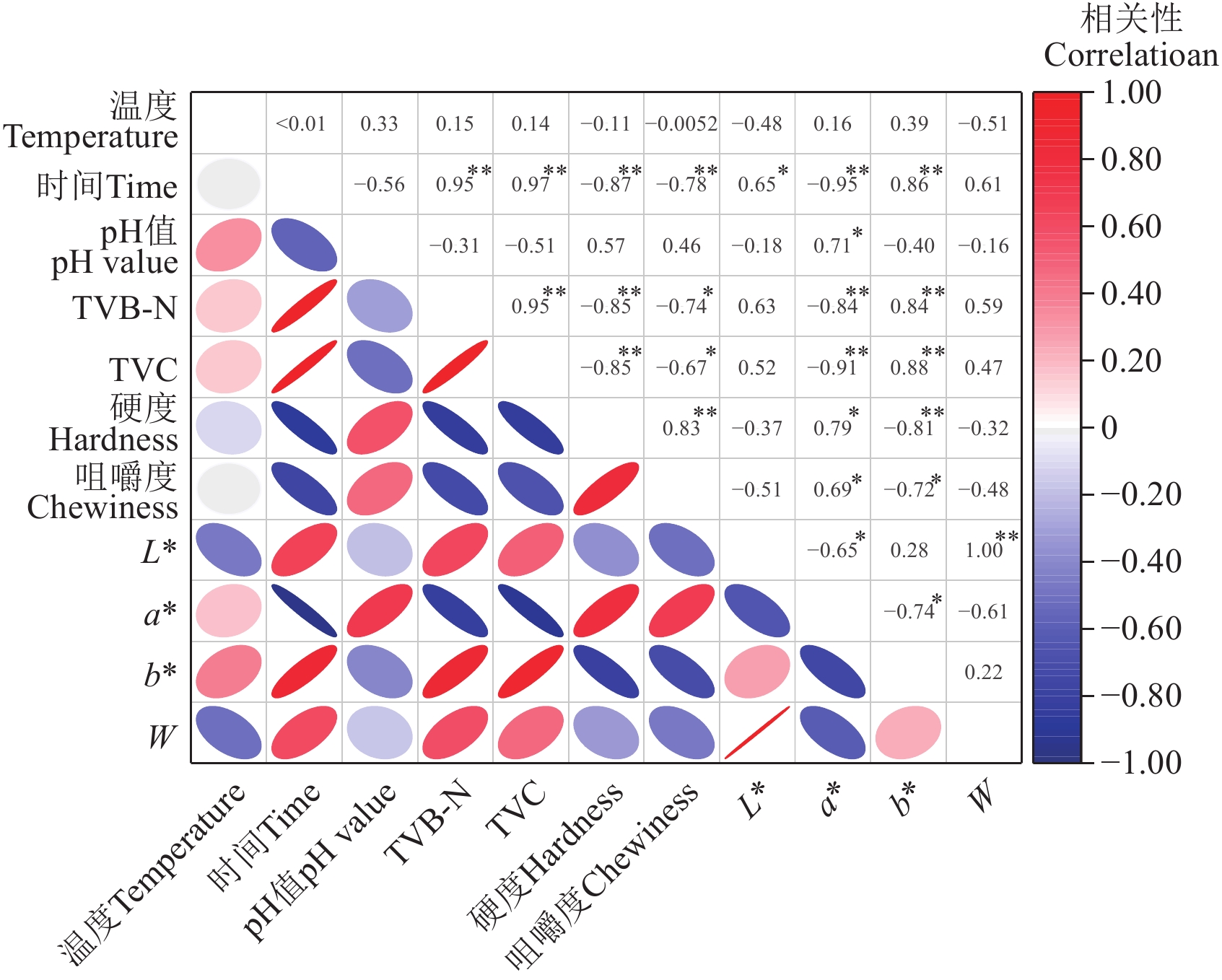
Pearson相关系数可以衡量不同指标间的线性相关程度。分析结果显示,贮藏温度、贮藏时间与TVB-N含量、TVC、硬度、咀嚼度、a*及b*等显著相关(图6)。
![]() 图 6 皮尔森相关性注:*表示P<0.05,**表示P<0.01,左下角椭圆形的大小表示相关性的程度,与右上角数值对应;椭圆形的方向表示正负相关性,方向为“/”表示正相关,反之为负相关。Figure 6. Pearson correlationNote: * denotes P<0.05, ** denotes P<0.01, the size of the ellipse in the lower left represents the degree of relevance, which corresponds to the value in the upper right corner; the direction of the ellipse represents a positive and negative correlation, and the direction "/" represents a positive correlation, and vice versa is a negative correlation.
图 6 皮尔森相关性注:*表示P<0.05,**表示P<0.01,左下角椭圆形的大小表示相关性的程度,与右上角数值对应;椭圆形的方向表示正负相关性,方向为“/”表示正相关,反之为负相关。Figure 6. Pearson correlationNote: * denotes P<0.05, ** denotes P<0.01, the size of the ellipse in the lower left represents the degree of relevance, which corresponds to the value in the upper right corner; the direction of the ellipse represents a positive and negative correlation, and the direction "/" represents a positive correlation, and vice versa is a negative correlation.此外,pH值与a*值的相关性系数为0.71(P<0.05),这是由于鱼体死后肌肉的pH值会影响高铁肌红蛋白的还原活性,导致鱼肉颜色发生变化[32]。TVC与TVB-N含量呈正相关(相关系数为0.95),TVC还与a*、b*、硬度和咀嚼度均有显著相关性,这可能是由于某些微生物(包括假单胞菌、光细菌和弧菌科)在低温贮藏期间产生氨(NH3)和甲胺(MA)等胺类物质,使得TVB-N含量上升,而且在微生物和内源酶的作用下,蛋白质发生降解,导致有机胺积累、肌肉纤维间隙变大,使得TVB-N含量、颜色和质构特性发生变化[33]。也即,贮藏温度越高,贮藏时间越长,鮰鱼品质劣变越严重。
2.8 菌群结构
为探究鮰鱼在冷藏过程中的菌群结构演替规律,本研究选择4 ℃作为贮藏温度进行分析。4 ℃是商业冷藏中常用的温度,研究其微生物组成对实际生产更具指导意义。为进一步分析腐败过程中的微生物群落变化,选择冷藏7 d的样品进行菌群结构分析。此时,鮰鱼的腐败程度较高,微生物群落趋于稳定,能够更好地反映优势腐败菌的组成。如表4所示。厚壁菌门、拟杆菌门和变形菌门是导致鮰鱼腐败的主要菌门。在腐败的鮰鱼肉中,共检测到19种菌科、20余类菌属和30余类菌种。如表5所示,黄杆菌科(Flavobacteriaceae)和气单胞菌科(Aeromonadaceae)是鮰鱼冷藏期间导致腐败的主要菌科。从属水平分析,鮰鱼冷藏期间检测丰度较高的菌属有类香味菌属Myroides(39.68%)、气单胞菌属Aeromonas(28.74%)、不动杆菌属Acinetobacter(16.33%)和希瓦氏菌属Shewanella(6.34%)。从种水平分析发现,相对丰度排名前3的腐败菌主要为类香味菌(Myroides profundi)、杀鲑气单胞菌(Aeromonas salmonicida)和腐败希瓦氏菌(Shewanella putrefaciens),其相对丰度分别为38.91%、28.74%和6.34%。腐败希瓦氏菌可从众多水产品中分离到,它可以产生H2S,与肌红蛋白结合,使鱼肉变绿,并产生臭鸡蛋的难闻气味[18, 34]。气单胞菌属能够显著增加水产品中的TVB-N和三甲胺的产量,这些物质的积累会加速其新鲜度的丧失[35]。这些微生物的代谢活动与鮰鱼在冷藏过程中的感官品质下降密切相关。
表 4 鮰鱼门水平的微生物组成Table 4. Microbial composition of channel catfish at phylum level门水平
Phylum level拟杆菌门
Bacteroidota厚壁菌门
Firmicutes变形菌门
Proteobacteria相对丰度
Relative abundance/%40.11 2.83 57.06 2.9 低温腐败菌的分离与形态学观察
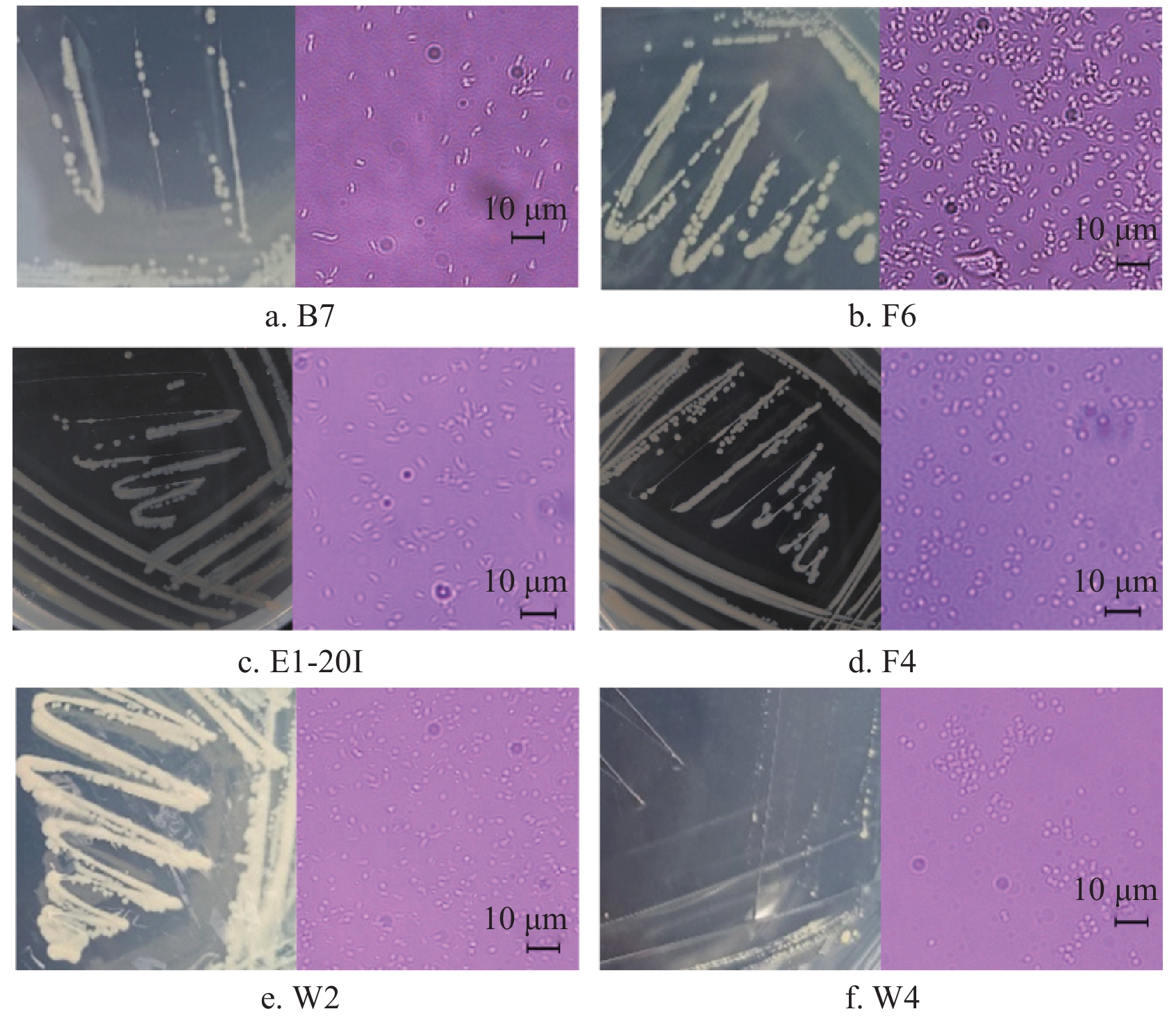
以4 ℃贮藏至腐败的鮰鱼为原料,根据菌落形态特征分离纯化出6株可在4 ℃条件下生长的低温腐败菌,对其进行形态学观察。如图7所示,为6株腐败菌的平板划线图片及镜检图片。通过形态学观察发现,这些菌落在平板培养基上呈现圆点状,呈现出乳白色,表面光滑且边缘整齐。进一步通过显微镜检观察,发现菌株的菌体形态主要为杆状或短杆状,且大多数菌体以单个形式存在。后续研究将结合分子生物学技术对这些菌株进行鉴定,以明确其分类地位及其致腐能力。
表 5 鮰鱼科、属和种水平的微生物组成Table 5. Microgenesis of catfish at family, genus and species level科水平
Family level相对丰度
Relative
abundance/%属水平
Genus level相对丰度
Relative
abundance/%种水平
Species level相对丰度
Relative
abundance/%气单胞菌科Aeromonadaceae 28.74 不动杆菌属Acinetobacter 16.33 Celticus不动杆菌Acinetobacter celticus 1.22 肠杆菌科Enterobacteriaceae 0.99 气单胞菌属Aeromonas 28.74 溶血不动杆菌Acinetobacter haemolyticus 5.90 黄杆菌科Flavobacteriaceae 39.68 索丝菌属Brochothrix 1.03 哈尔滨不动杆菌Acinetobacter harbinensis 5.00 李斯特菌科Listeriaceae 1.03 布丘氏菌属Buttiauxella 0.99 约氏不动杆菌Acinetobacter johnsonii 4.14 莫拉氏菌科Moraxellaceae 16.50 摩根菌属Morganella 1.32 杀鲑气单胞菌Aeromonas salmonicida 28.74 摩根菌科Morganellaceae 1.56 类香味菌属Myroides 39.68 耐冷性摩根菌Morganella psychrotolerans 1.32 假单胞菌科Pseudomonadaceae 1.16 假单胞菌属Pseudomonas 1.16 类香味菌Myroides profundi 38.91 希瓦氏菌科Shewanellaceae 6.34 沙雷氏菌属Serratia 1.28 产氮假单胞菌Pseudomonas azotoformans 1.14 漫游球菌科Vagococcaceae 1.27 希瓦氏菌属Shewanella 6.34 变形斑沙雷氏菌Serratia proteamaculans 1.28 耶尔森菌科Yersiniaceae 1.28 漫游球菌属Vagococcus 1.27 腐败希瓦氏菌Shewanella putrefaciens 6.34 注:此表为相对丰度前10的微生物组成。 Note: The table shows the top 10 microbial compositions in relative abundance. 2.10 16S rDNA测序结果及致腐能力
菌株分子鉴定结果见表6。以腐败鮰鱼肌肉为原料,本研究共分离鉴定出6株菌,包括1株嗜冷假单胞菌(B7为Pseudomonas psychrophila)、1株莓实假单胞菌(F6为Pseudomonas fragi)、1株希瓦氏菌(E1-20I为Shewanella baltica)、2株维氏气单胞菌(F4和W2为Aeromonas veronii)以及1株约氏不动杆菌(W4为Acinetobacter johnsonii)。筛选结果与2.8中鮰鱼微生物多样性研究结果一致。其中,假单胞菌是水产品中常见的嗜冷性致腐菌,可以产生高活性的蛋白酶和脂肪酶,对鱼类的变质起着重要作用[36]。希瓦氏菌在低温条件下仍能生长繁殖,导致水产品中的肌肉蛋白发生分解,这不仅影响水产品感官品质,还会产生腐胺、尸胺等有害物质[37]。维氏气单胞菌被广泛认为是一种对人类健康有危害的致病菌[38]。不动杆菌属是一种需氧的革兰氏阴性细菌,是引发人类社区和医院感染的重要致病菌[39]。这些菌株的筛选为进一步研究鮰鱼腐败机制及优势腐败菌的致腐能力提供了重要材料。
表 6 细菌16S rDNA序列分析结果及致腐能力Table 6. Results of 16S rDNA sequence analysis for bacteria and decay-causing ability菌株
编号
Strain number最高相似
参考菌株
Highest similar reference strain核苷酸
序列号
GeneBank同源性
Homology/%YTVB-N/CFU/
(10−8mg·CFU−1)B7 Pseudomonas psychrophila CP049044.1 100.00 4.85 ± 0.94c F6 Pseudomonas fragi MH463554.1 99.93 6.73 ± 2.10bc E1-20I Shewanella baltica MT516290.1 100.00 7.08 ± 0.75b F4 Aeromonas veronii MG063203.1 100.00 9.66 ± 1.62a W2 Aeromonas veronii MT226399.1 99.93 1.08 ± 0.21d W4 Acinetobacter johnsonii CP068206.1 99.85 2.27 ± 0.20d 注:YTVB-N/CFU表示致腐能力,小写字母不同,表示致腐能力差异显著(P<0.05) Notes: YTVB-N/CFU indicates the ability to cause rot, differences in lower case letters indicate significant differences in decay-causing ability (P<0.05). 通过腐败代谢产物产量因子YTVB-N/CFU表示致腐能力。由表6可知,6株SSOs的YTVB-N/CFU不同,表明不同腐败菌导致鮰鱼腐败能力存在差异,这6株低温腐败菌的致腐能力大小依次为:F4、E1-20I、F6、B7、W4、W2,其中维氏气单胞菌(Aeromonas veronii)和希瓦氏菌(Shewanella baltica)的致腐能力最强,约氏不动杆菌(Acinetobacter johnsonii)的致腐能力相对较弱。已有研究表明气单胞菌属和希瓦氏菌属是水产中常见的优势腐败菌,在草鱼[40]、南美白对虾[41]中均作为优势腐败菌被发现。希瓦氏菌属和气单胞菌属是具有高胺代谢能力的革兰氏阴性菌,被认为是水产品中的主要腐败微生物,可以将三甲胺-N-氧化物(TMAO)转化为三甲胺(TMA),这一过程不仅导致鱼类产生难闻的气味,还加速了鱼类的腐烂变质[42]。
2.11 SSOs的生长动力学分析
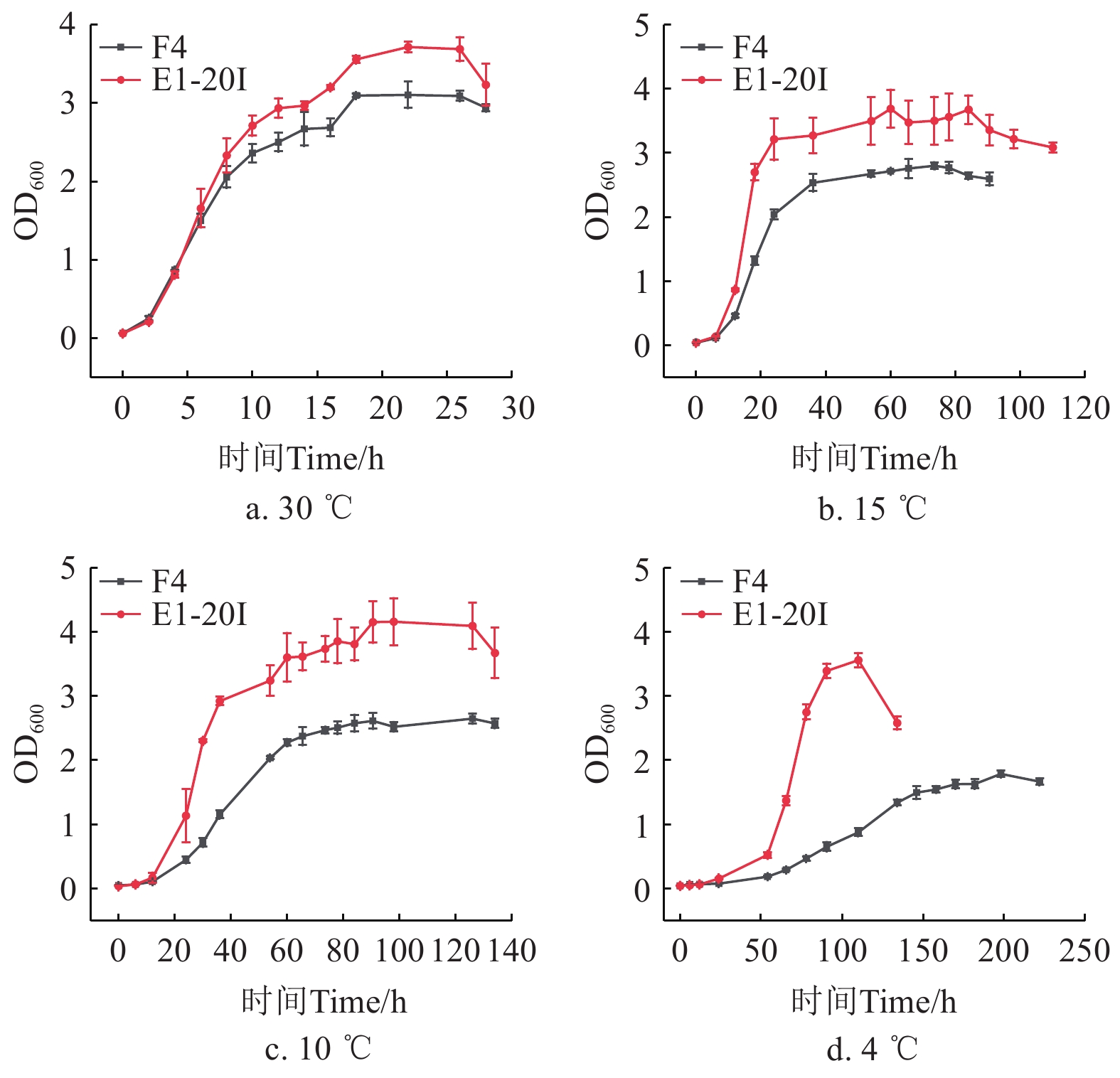
为探究腐败菌在不同温度条件下的生长繁殖情况,选择致腐能力最强的2株菌(F4和E1-20I),测定其在不同温度下的生长曲线。如图8所示,在30 ℃培养2 h后进入对数期,18 h后增长缓慢,为稳定期,26 h后进入衰退期。采用修正的Gompertz模型对SSOs的生长曲线进行拟合,得到的生长动力学参数见表7。修正的Gompertz方程拟合的2株腐败菌在30、15、10和4 ℃培养下的生长曲线拟合系数R2大于0.95,具有较高的拟合度,能够很好的反映菌株的生长情况。随着培养温度的下降,2种腐败菌的延滞时间λ显著延长,最大比生长速率μmax明显降低,表明低温条件下微生物的生长繁殖速度减缓。数据显示,E1-20I的最大比生长速率μmax始终高于F4,说明希瓦氏菌在低温环境下的适应能力更强。这可能是由于希瓦氏菌细胞膜脂肪酸代谢相关酶的表达量较高,且细胞膜蛋白组成和含量增加,使其壁膜微观结构更加致密,从而增强了低温适应能力[43]。另一方面,气单胞菌属虽然最大比生长速率μmax较低,但其良好的菌膜形成能力为其提供多种代谢所需的营养物质,并保护其免受环境胁迫的影响,从而增强了其对不利环境的耐受性[44]。综上所述,这2株菌具有在低温条件下生长的能力,但E1-20I的低温适应性更为突出。
![]() 图 8 腐败菌在不同温度下的生长曲线注:OD600表示菌液在600 nm波长处的吸光值,F4和E1-20I分别为维氏气单胞菌和希瓦氏菌,下同。Figure 8. Growth curves of spoilage bacteria at different temperaturesNotes: OD600 represents the light absorption value of the bacterial solution at the wavelength of 600 nm, F4 and E1-20I stand for Aeromonas veronii and Shewanella baltica, respectively, the same below.表 7 不同培养温度下优势腐败菌的生长动力学参数Table 7. Growth kinetic parameters of specific spoilage organisms at different incubation temperatures
图 8 腐败菌在不同温度下的生长曲线注:OD600表示菌液在600 nm波长处的吸光值,F4和E1-20I分别为维氏气单胞菌和希瓦氏菌,下同。Figure 8. Growth curves of spoilage bacteria at different temperaturesNotes: OD600 represents the light absorption value of the bacterial solution at the wavelength of 600 nm, F4 and E1-20I stand for Aeromonas veronii and Shewanella baltica, respectively, the same below.表 7 不同培养温度下优势腐败菌的生长动力学参数Table 7. Growth kinetic parameters of specific spoilage organisms at different incubation temperatures温度
Temperature/℃菌株编号
Strain numberN0 Nmax μmax/h−1 λ/h R2 30 E1-20I 0.064 3.495 0.361 1.834 0.981 F4 0.065 3.013 0.299 1.386 0.988 15 E1-20I 0.046 3.429 0.353 9.671 0.981 F4 0.043 2.705 0.147 9.299 0.997 10 E1-20I 0.040 3.875 0.128 13.735 0.981 F4 0.051 2.621 0.066 19.344 0.999 4 E1-20I 0.045 3.216 0.129 53.544 0.951 F4 0.045 1.822 0.017 54.753 0.994 注:N0为初始OD600值,Nmax为最大OD600值,μmax为最大比生长速率,λ为延滞期,R2为拟合系数。 Notes: N0 is the initial OD600 value, Nmax is the maximum OD600 value. μmax is the maximum specific growth rate, λ is the demurrage period, R2 is the fitting coefficient. 3. 结 论
通过对鮰鱼的感官评价、理化指标、微生物群落结果及腐败菌的致腐能力进行研究,得到如下结论:
1)在4和10 ℃冷藏过程中,鮰鱼发生不同程度的腐败。冷藏期间,pH值呈现先下降后上升的趋势,挥发性盐基氮含量、菌落总数、亮度(L*)、黄蓝值(b*)及白度(W)显著上升,而硬度、咀嚼度和红绿值(a*)下降。综合各项指标表明,4 ℃贮藏条件下,鮰鱼在2 d内品质保持较好,适合短期贮藏。
2)通过高通量测序分析发现,腐败鮰鱼肉中涉及20余类菌属,其中类香味菌属(Myroides)的相对丰度最高,其次为气单胞菌属(Aeromonas)、不动杆菌属(Acinetobacter)和希瓦氏菌属(Shewanella)。这些菌属在冷藏过程中发挥了重要作用。
3)以腐败鮰鱼为对象,筛选并鉴定了6株腐败菌,包括嗜冷假单胞菌(Pseudomonas psychrophila)、莓实假单胞菌(Pseudomonas fragi)、维氏气单胞菌(Aeromonas veronii)、希瓦氏菌(Shewanella baltica)以及约氏不动杆菌(Acinetobacter johnsonii)。试验表明,希瓦氏菌和维氏气单胞菌的致腐能力最强,且在低温条件下生长良好,是鮰鱼冷藏期间的优势腐败菌。
综上所述,4 ℃冷藏2 d内鮰鱼品质较好,希瓦氏菌属(Shewanella)和气单胞菌属(Aeromonas)为鮰鱼冷藏期间的优势腐败菌。本研究为鮰鱼的保鲜技术优化提供了科学依据,并为农产品加工领域的品质控制提供了经验参考。未来研究可进一步探索这些腐败菌的代谢机制,并开发针对性的抑菌策略,以延长水产品的货架期并保障食品安全。
-
图 1 鮰鱼在不同温度贮藏期间的感官评分
注:大写字母不同表示相同贮藏时间内,不同贮藏温度差异显著;小写字母不同表示相同贮藏温度,不同贮藏时间差异显著(P<0.05),下同。
Figure 1. Sensory scores of channel catfish during storage at different temperatures
Note: The difference of capital letters means that the difference of storage temperature is significant in the same storage time. Different lowercase letters indicate the same storage temperature, and the difference is significant for different storage times (P < 0.05), the same below.
图 6 皮尔森相关性
注:*表示P<0.05,**表示P<0.01,左下角椭圆形的大小表示相关性的程度,与右上角数值对应;椭圆形的方向表示正负相关性,方向为“/”表示正相关,反之为负相关。
Figure 6. Pearson correlation
Note: * denotes P<0.05, ** denotes P<0.01, the size of the ellipse in the lower left represents the degree of relevance, which corresponds to the value in the upper right corner; the direction of the ellipse represents a positive and negative correlation, and the direction "/" represents a positive correlation, and vice versa is a negative correlation.
图 8 腐败菌在不同温度下的生长曲线
注:OD600表示菌液在600 nm波长处的吸光值,F4和E1-20I分别为维氏气单胞菌和希瓦氏菌,下同。
Figure 8. Growth curves of spoilage bacteria at different temperatures
Notes: OD600 represents the light absorption value of the bacterial solution at the wavelength of 600 nm, F4 and E1-20I stand for Aeromonas veronii and Shewanella baltica, respectively, the same below.
表 1 感官评定指标
Table 1 Sensory evaluation index
评分项目
Scoring item感官指标
Sensory index分数
Score外观
Appearance色泽均匀,表面富有光泽 11~15 色泽较暗淡,基本无光泽 6~10 暗淡无光泽 1~5 气味
Smell有较淡的鲜美鱼腥味,无不良气味 11~15 腥味明显,略有臭异味 6~10 有强烈的腥臭味 1~5 质地
Texture肌肉坚实,有弹性,外形保持完好 11~15 肌肉松软,弹性较差,外形保持较完好 6~10 肌肉软烂,无弹性,外形保持较差 1~5 黏液
Mucus透明,无黏液 11~15 较少黏液,乳白色 6~10 较多黏液,黄色 1~5 表 2 鮰鱼在4 ℃下贮藏期间色泽的变化
Table 2 Changes of color of channel catfish during storage at 4 ℃
时间
Time/dL* a* b* W 0 49.56±2.90b −2.37±0.20a 0.46±0.33b 49.50±2.89b 2 49.81±1.48b −3.16±0.24b 0.74±0.60b 49.70±1.47b 4 50.37±1.00b −3.49±0.25bc 2.34±0.50a 50.19±0.98b 6 51.70±3.17ab −3.68±0.34c 2.38±0.63a 51.49±3.16ab 8 53.46±1.33a −4.25±0.44d 2.63±0.76a 53.19±1.37a 注:L*表示亮度,a*表示红绿值、b*表示黄蓝值,W表示白度,下同。 Note: L* represents lightness, a* represents the red-green value, b* represents the yellow-blue value, and W represents the whiteness, the same below. 表 3 鮰鱼在10 ℃下贮藏期间色泽的变化
Table 3 Changes of color of channel catfish during storage at 10 ℃
时间
Time/dL* a* b* W 0 49.56±2.90abc −2.37±0.20a 0.46±0.33d 49.50±2.89b 2 48.05±3.54bc −2.53±0.75ab 2.14±1.05c 47.93±3.50ab 4 46.97±0.84c −3.40±0.37bc 2.81±1.12bc 46.78±0.84ab 6 50.57±2.08ab −3.77±0.75c 3.72±1.01ab 50.27±2.07a 8 51.44±1.43a −3.85±1.10c 5.01±1.28a 51.00±1.42a 表 4 鮰鱼门水平的微生物组成
Table 4 Microbial composition of channel catfish at phylum level
门水平
Phylum level拟杆菌门
Bacteroidota厚壁菌门
Firmicutes变形菌门
Proteobacteria相对丰度
Relative abundance/%40.11 2.83 57.06 表 5 鮰鱼科、属和种水平的微生物组成
Table 5 Microgenesis of catfish at family, genus and species level
科水平
Family level相对丰度
Relative
abundance/%属水平
Genus level相对丰度
Relative
abundance/%种水平
Species level相对丰度
Relative
abundance/%气单胞菌科Aeromonadaceae 28.74 不动杆菌属Acinetobacter 16.33 Celticus不动杆菌Acinetobacter celticus 1.22 肠杆菌科Enterobacteriaceae 0.99 气单胞菌属Aeromonas 28.74 溶血不动杆菌Acinetobacter haemolyticus 5.90 黄杆菌科Flavobacteriaceae 39.68 索丝菌属Brochothrix 1.03 哈尔滨不动杆菌Acinetobacter harbinensis 5.00 李斯特菌科Listeriaceae 1.03 布丘氏菌属Buttiauxella 0.99 约氏不动杆菌Acinetobacter johnsonii 4.14 莫拉氏菌科Moraxellaceae 16.50 摩根菌属Morganella 1.32 杀鲑气单胞菌Aeromonas salmonicida 28.74 摩根菌科Morganellaceae 1.56 类香味菌属Myroides 39.68 耐冷性摩根菌Morganella psychrotolerans 1.32 假单胞菌科Pseudomonadaceae 1.16 假单胞菌属Pseudomonas 1.16 类香味菌Myroides profundi 38.91 希瓦氏菌科Shewanellaceae 6.34 沙雷氏菌属Serratia 1.28 产氮假单胞菌Pseudomonas azotoformans 1.14 漫游球菌科Vagococcaceae 1.27 希瓦氏菌属Shewanella 6.34 变形斑沙雷氏菌Serratia proteamaculans 1.28 耶尔森菌科Yersiniaceae 1.28 漫游球菌属Vagococcus 1.27 腐败希瓦氏菌Shewanella putrefaciens 6.34 注:此表为相对丰度前10的微生物组成。 Note: The table shows the top 10 microbial compositions in relative abundance. 表 6 细菌16S rDNA序列分析结果及致腐能力
Table 6 Results of 16S rDNA sequence analysis for bacteria and decay-causing ability
菌株
编号
Strain number最高相似
参考菌株
Highest similar reference strain核苷酸
序列号
GeneBank同源性
Homology/%YTVB-N/CFU/
(10−8mg·CFU−1)B7 Pseudomonas psychrophila CP049044.1 100.00 4.85 ± 0.94c F6 Pseudomonas fragi MH463554.1 99.93 6.73 ± 2.10bc E1-20I Shewanella baltica MT516290.1 100.00 7.08 ± 0.75b F4 Aeromonas veronii MG063203.1 100.00 9.66 ± 1.62a W2 Aeromonas veronii MT226399.1 99.93 1.08 ± 0.21d W4 Acinetobacter johnsonii CP068206.1 99.85 2.27 ± 0.20d 注:YTVB-N/CFU表示致腐能力,小写字母不同,表示致腐能力差异显著(P<0.05) Notes: YTVB-N/CFU indicates the ability to cause rot, differences in lower case letters indicate significant differences in decay-causing ability (P<0.05). 表 7 不同培养温度下优势腐败菌的生长动力学参数
Table 7 Growth kinetic parameters of specific spoilage organisms at different incubation temperatures
温度
Temperature/℃菌株编号
Strain numberN0 Nmax μmax/h−1 λ/h R2 30 E1-20I 0.064 3.495 0.361 1.834 0.981 F4 0.065 3.013 0.299 1.386 0.988 15 E1-20I 0.046 3.429 0.353 9.671 0.981 F4 0.043 2.705 0.147 9.299 0.997 10 E1-20I 0.040 3.875 0.128 13.735 0.981 F4 0.051 2.621 0.066 19.344 0.999 4 E1-20I 0.045 3.216 0.129 53.544 0.951 F4 0.045 1.822 0.017 54.753 0.994 注:N0为初始OD600值,Nmax为最大OD600值,μmax为最大比生长速率,λ为延滞期,R2为拟合系数。 Notes: N0 is the initial OD600 value, Nmax is the maximum OD600 value. μmax is the maximum specific growth rate, λ is the demurrage period, R2 is the fitting coefficient. -
[1] 代云云,袁永明,袁媛,等. 中国鮰鱼产业发展现状及趋势分析[J]. 湖南农业科学,2019(2):89-92. DAI Yunyun, YUAN Yongming, YUAN Yuan, et al. Current and future studies on squid industry in China[J]. Hunan Agricultural Sciences, 2019(2): 89-92. (in Chinese with English abstract)
[2] XIA R, HAO Q, XIE Y D, et al. Effects of dietary Saccharomyces cerevisiae on growth, intestinal and liver health, intestinal microbiota and disease resistance of channel catfish (Ictalurus punctatus)[J]. Aquaculture Reports, 2022, 24: 101157. DOI: 10.1016/j.aqrep.2022.101157
[3] 陈德智,陈俊男,刘真熙,等. 不同煮制条件对斑点叉尾鮰鱼肉食用品质的影响[J]. 科学养鱼,2024,(2):80-82. [4] ZHUANG S, ZHANG X Y, LUO Y K, et al. LC-MS/MS-based peptidomics and metabolomics reveal different metabolic patterns of common spoilage bacteria in grass carp flesh[J]. Food Bioscience, 2024, 60: 104480. DOI: 10.1016/j.fbio.2024.104480
[5] BAPTISTA C R, OLIVEIRA A B R, CAMARA A A, et al. Chilled Pacu (Piaractus mesopotamicus) fillets: Modeling Pseudomonas spp. and psychrotrophic bacteria growth and monitoring spoilage indicators by 1H NMR and GC-MS during storage[J]. International Journal of Food Microbiology, 2024, 415: 110645. DOI: 10.1016/j.ijfoodmicro.2024.110645
[6] LI Y, JIA S L, HONG H, et al. Assessment of bacterial contributions to the biochemical changes of chill-stored blunt snout bream (Megalobrama amblycephala) fillets: Protein degradation and volatile organic compounds accumulation[J]. Food Microbiology, 2020, 91: 103495. DOI: 10.1016/j.fm.2020.103495
[7] 周慧,盖园明,徐超,等. 基于16S rDNA测序及培养基法探究虹鳟鱼贮藏优势腐败菌[J]. 微生物学杂志,2021,41(1):25-32. ZHOU Hui, GAI Yuanming, XU Chao, et al. Based on 16S rDNA sequencing and culture medium method to explore the dominant spoilage bacteria in storage of rainbow trout[J]. Journal of Microbiology, 2021, 41(1): 25-32. (in Chinese with English abstract)
[8] 柴庭庭,朱星橦,柴保臣,等. 大黄鱼优势腐败菌的致腐能力及对挥发性风味的影响[J]. 中国食品学报,2024,24(6):329-340 CHAI Tingting, ZHU Xingzhuang, CHAI Baochen, et al. The spoilage ability of dominant spoilage bacteria in large yellow croaker and their impact on volatile flavor[J]. Journal of Chinese Institute of Food Science and Technology, 2024, 24(6): 329-340. (in Chinese with English abstract)
[9] 刘洋帆,王迪,李春生,等. 冷藏鲣鱼优势腐败产胺菌分离鉴定及其致腐产胺能力分析[J]. 食品工业科技,2022,43(23):168-175. LIU Yangfan, WANG Di, LI Chunsheng, et al. Isolation and identification of dominant spoilage biogenic amine producing bacteria from refrigerated Skipjack Tuna and analysis of their production ability[J]. Science and Technology of Food Industry, 2022, 43(23): 168-175. (in Chinese with English abstract).
[10] 杨永安,李静静,刘建福,等. 不同温度波动对冻藏三文鱼色差和质构的影响[J]. 食品与发酵工业,2021,47(10):145-150. YANG Yongan, LI Jingjing, LIU Jianfu, et al. The effect of temperature fluctuation on color difference and texture of frozen salmon[J]. Food and Fermentation Industries, 2021, 47(10): 145-150. (in Chinese with English abstract)
[11] 卜俊芝,徐迅,王玉宝,等. 真空低温慢煮对三文鱼品质及脂质组成的影响[J]. 农业工程学报,2025,41(4):318-330. BU Junzhi, XU Xun, WANG Yubao, et al. Effects of sous-vide cooking on the quality and lipid composition of salmon[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2025, 41(4): 318-330. (in Chinese with English abstract)
[12] 陈新文,赵颖莹,孟嫚,等. 超声辅助真空湿法腌制对臭鳜鱼品质及风味的影响[J]. 食品安全导刊,2024(24):131-137,165. CHEN Xinwen, ZHAO Yingying, MENG Man, et al. Effects of ultrasound assisted vacuum wet curing on the quality and flavor of stinky mandarin fish[J]. China Food Safety Magazine, 2024(24): 131-137,165. (in Chinese with English abstract)
[13] 蒋金辰,徐琰斐,单建军,等. 鱼菜共生系统中乳酸菌的筛选及其发酵矿化应用[J]. 农业工程学报,2024,40(11):119-127. JIANG Jinchen, XU Yanfei, SHAN Jianjun, et al. Screening lactic acid bacteria for fermentation and mineralization in aquaponic system[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2024, 40(11): 119-127. (in Chinese with English abstract)
[14] 梁嘉平,姚磊磊,陈刚. 水炭耦合对芦笋根际土壤微生物群落结构的影响[J]. 农业工程学报,2025,41(1):135-143. LIANG Jiaping, YAO Leilei, CHEN Gang. Effects of water-biochar coupling on asparagus rhizosphere soil microbial communitystructure[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2025, 41(1): 135-143. (in Chinese with English abstract)
[15] 范力艺,范群艳,胡嘉淼,等. 基于高通量测序技术的燕窝罐头腐败嗜热菌分析[J]. 农业工程学报,2022,38(3):295-303. FAN Liyi, FAN Qunyan, HU Jiamiao, et al. Analysis of spoilage thermophilic bacteria in canned edible bird nest based on high-throughput sequencing technology[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2022, 38(3): 295-303. (in Chinese with English abstract)
[16] 梁伟兴,迟淑艳,杨烜懿,等. 复合蛋白源对杂交石斑鱼生长和肠道菌群的影响[J]. 农业工程学报,2023,39(4):256-266. LIANG Weixing, CHI Shuyan, YANG Xuanyi, et al. Effects of complex protein sources on the growth and intestinal microflora of hybrid grouper[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2023, 39(4): 256-266. (in Chinese with English abstract)
[17] 徐文欢,吴若菡,李采婵,等. 传统虾酱中酵母菌分离鉴定及碳源利用特性[J]. 中国食品学报,2021,21(4):303-309. XU Wenhuan, WU Ruohan, LI Caichan, et al. Separation and identification of yeast from traditional shrimp paste and their carbon utilization characteristics[J]. Journal of Chinese Institute of Food Science and Technology, 2021, 21(4): 303-309. (in Chinese with English abstract)
[18] 高天麒,汪超,汪兰,等. 冷藏鮰鱼中腐败菌的分离鉴定及腐败能力测定[J]. 肉类研究,2023,37(3):1-6. GAO Tianlin, WANG Chao, WANG Lan, et al. Isolation, identification and spoilage ability of dominant spoilage bacteria from Channel Catfish[J]. Meat Research, 2023, 37(3): 1-6. (in Chinese with English abstract)
[19] 郑瑞生,邹菊琴,金兴珏,等. 即食鲍鱼特定腐败菌的鉴定及其致腐能力研究[J]. 中国食品学报,2021,21(7):307-312. ZHENG Ruisheng, ZOU Juqin, JIN Xingjue, et al. Identification of specific spoilage organism in ready-to-eat abalone and studies on its spoilage potential[J]. Journal of Chinese Institute of Food Science and Technology, 2021, 21(7): 307-312. (in Chinese with English abstract)
[20] SOYUL L, AREUM H, JAE Y Y, et al. Growth evaluation of Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes in fresh fruit and vegetable juices via predictive modeling[J]. LWT-Food Science and Technology, 2022, 162: 113485 DOI: 10.1016/j.lwt.2022.113485
[21] 张艳凌,向俊飞,朱亚军,等. 鲜活和死后小龙虾的冷藏特性比较[J]. 食品科学,2022,43(1):206-212. ZHANG Yanling, XIANG Junfei, ZHU Yajun, et al. Comparison of quality characteristics of live and dead crayfish during cold storage[J]. Food Science, 2022, 43(1): 206-212. (in Chinese with English abstract)
[22] LI Y Y, JIANG S, ZHU Y W, et al. Effect of different drying methods on the taste and volatile compounds, sensory characteristics of Takifugu obscurus[J]. Food Science and Human Wellness, 2023, 12(1): 223-232. DOI: 10.1016/j.fshw.2022.07.012
[23] 汤元睿,谢 晶,李念文,等. 不同冷链物流过程对金枪鱼品质及组织形态的影响[J]. 农业工程学报,2014,30(5):285-292. TANG Yuanrui, XIE Jing, LI Nianwen, et al. Effects of different cold chain logistics situations on quality and microstructure of tuna (Thunnus obesus) fillets[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2014, 30(5): 285-292. (in Chinese with English abstract)
[24] LAN W Q, HU X Y, SUN X H, et al. Effect of the number of freeze-thaw cycles number on the quality of pacific white shrimp (Litopenaeus vannamei): An emphasis on moisture migration and microstructure by LF-NMR and SEM[J]. Aquaculture and Fisheries, 2020, 5(4): 193-200. DOI: 10.1016/j.aaf.2019.05.007
[25] 辜雪冬,孙术国,杨飞艳,等. 冰温或冷藏对牦牛肉贮藏品质及水分迁移的影响[J]. 农业工程学报,2019,35(16):343-350. GU Xuedong, SUN Shuguo, YANG Feiyan, et al. Effects of ice temperature or chilled storage on quality of yak meat and moisture migration[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(16): 343-350. (in Chinese with English abstract)
[26] WU Z Y, MA W R, XIAN Z J, et al. The impact of quick-freezing methods on the quality, moisture distribution and microstructure of prepared ground pork during storage duration[J]. Ultrasonics Sonochemistry, 2021, 78: 105707. DOI: 10.1016/j.ultsonch.2021.105707
[27] 孙光全,冯耀泽,朱明,等. 面向长途冷链下不同冷冻方式对大口黑鲈品质的影响[J]. 农业工程学报,2023,39(24):316-326. SUN Guangquan, FENG Yaoze, ZHU Ming, et al. Effects of different freezing methods on the quality of Micropterus salmoides oriented to long-distance cold chain[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2023, 39(24): 316-326. (in Chinese with English abstract)
[28] 蓝蔚青,张炳杰,周大鹏,等. 超声联合微酸性电解水处理对真空包装海鲈鱼冷藏期间品质变化的影响[J]. 食品科学,2022,43(5):62-68. LAN Weiqing, ZHANG Bingjie, ZHOU Dapeng, et al. Effect of ultrasonic combined with slightly acidic electrolyzed water treatment on quality changes of vacuum-packaged sea bass (Lateolabrax japonicas) during refrigerated storage[J]. Food Science, 2022, 43(5): 62-68. (in Chinese with English abstract)
[29] YANG X R, MEI J, XIE J. Effects of different slaughtering methods on the biochemical characteristics and quality changes of tilapia (Oreochromis niloticus) during cold storage[J]. Food Chemistry: X, 2024, 24: 101951. DOI: 10.1016/j.fochx.2024.101951
[30] LI B B, LIU S J, CHEN X T, et al. Dynamic changes in the microbial composition and spoilage characteristics of refrigerated large yellow croaker (Larimichthys crocea) during storage[J]. Foods, 2023, 12(21): 3994. DOI: 10.3390/foods12213994
[31] SHI L, YIN T, XIONG G Q, et al. Microstructure and physicochemical properties: Effect of pre-chilling and storage time on the quality of Channel catfish during frozen storage[J]. LWT-Food Science and Technology, 2020, 130: 109606. DOI: 10.1016/j.lwt.2020.109606
[32] CHAUHAN S, ENGLAND M E. Postmortem glycolysis and glycogenolysis: insights from species comparisons[J]. Meat Science, 2018, 144: 118-126. DOI: 10.1016/j.meatsci.2018.06.021
[33] LU X, ZHANG Y M, ZHU L X, et al. Effect of super chilled storage on shelf life and quality characteristics of M. longissimus lumborum from Chinese Yellow cattle[J]. Meat Science, 2018, 149: 79-84.
[34] 刘舒彦,熊光权,李海蓝,等. 加州鲈优势腐败菌的分离鉴定[J]. 现代食品科技,2019,35(11):120-125,230. LIU Shuyan, XIONG Guangquan, LI Hailan, et al. Isolation and identification of the dominant spoilage bacteria from california bass (Micropterus salmoides)[J]. Modern Food Science and Technology, 2019, 35(11): 120-125,230. (in Chinese with English abstract)
[35] LIU L, YAN Y P, FENG L F, et al. Quorum sensing asal mutants affect spoilage phenotypes, motility, and biofilm formation in a marine fish isolate of Aeromonas salmonicida[J]. Food Microbiology, 2018, 76: 40-51. DOI: 10.1016/j.fm.2018.04.009
[36] JIA S L, JIA Z F, AN J, et al. Insights into the fish protein degradation induced by the fish-borne spoiler Pseudomonas psychrophila and Shewanella putrefaciens: From whole genome sequencing to quality changes[J]. International Journal of Food Microbiology, 2024, 416: 110675. DOI: 10.1016/j.ijfoodmicro.2024.110675
[37] FU L L, WANG C, LIU N N, et al. Quorum sensing system-regulated genes affect the spoilage potential of Shewanella baltica[J]. Food Research International, 2018, 107: 1-9. DOI: 10.1016/j.foodres.2018.01.067
[38] 熊广泽,张怡晴,刘子琦,等. 维氏气单胞菌对斑马鱼胚胎发育的阻滞[J]. 水产科学,2024,43(4):630-639. XIONG Guangze, ZHANG Yiqing, LIU Ziqi, et al. Inhibition of embryonic development of zebrafish by Aeromonas veronii[J]. Fisheries Science, 2024, 43(4): 630-639. (in Chinese with English abstract)
[39] KOLK D V J, ENDIMIANI A, GRAUBNER C, et al. Acinetobacter in veterinary medicine, with an emphasis on Acinetobacter baumannii[J]. Journal of Global Antimicrobial Resistance, 2019, 16: 59-71. DOI: 10.1016/j.jgar.2018.08.011
[40] 温欣怡,李晴,梁释介,等. 草鱼不同部位肉在冷藏过程中的品质变化[J]. 广东海洋大学学报,2024,44(5):85-95. WEN Xinyi, LI Qing, LIANG Shijie, et al. Quality changes in different parts of Ctenopharyngodon idellu meat during cold storage[J]. Journal of Guangdong Ocean University, 2024, 44(5): 85-95. (in Chinese with English abstract)
[41] QIAN Y, YE J, YANG S, et al. Evaluation of the spoilage potential of Shewanella putrefaciens, Aeromonas hydrophila, and Aeromonas sobria isolated from spoiled Pacific white shrimp (Litopenaeus vannamei) during cold storage[J]. Journal of Food Safety, 2018, 38(6): e12550. DOI: 10.1111/jfs.12550
[42] LOU X W, ZHAI D D, YANG H S. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage[J]. Food Control, 2021, 123: 107697. DOI: 10.1016/j.foodcont.2020.107697
[43] 杨胜平,章缜,程颖,等. 培养温度对腐败希瓦氏菌DSM6067生长动力学及细胞膜理化特性的影响[J]. 中国食品学报,2020,20(2):228-236 YANG Shengping, ZHANG Zhen, CHENG Ying, et al. Effects of culture temperature on the growth parameters of Shewanella putrefaciens DSM6067 and its membrane physic-chemical properties[J]. Journal of Chinese Institute of Food Science and Technology, 2020, 20(2): 228-236. (in Chinese with English abstract)
[44] LI Y L, WANG T Q, SUN Z D, et al. Inhibition mechanism of crude lipopeptide from Bacillus subtilis against Aeromonas veronii growth, biofilm formation, and spoilage of channel catfish flesh[J]. Food Microbiology, 2024, 120: 104489. DOI: 10.1016/j.fm.2024.104489




 下载:
下载:
 下载:
下载:












